MaxCyte IPO Presentation Deck
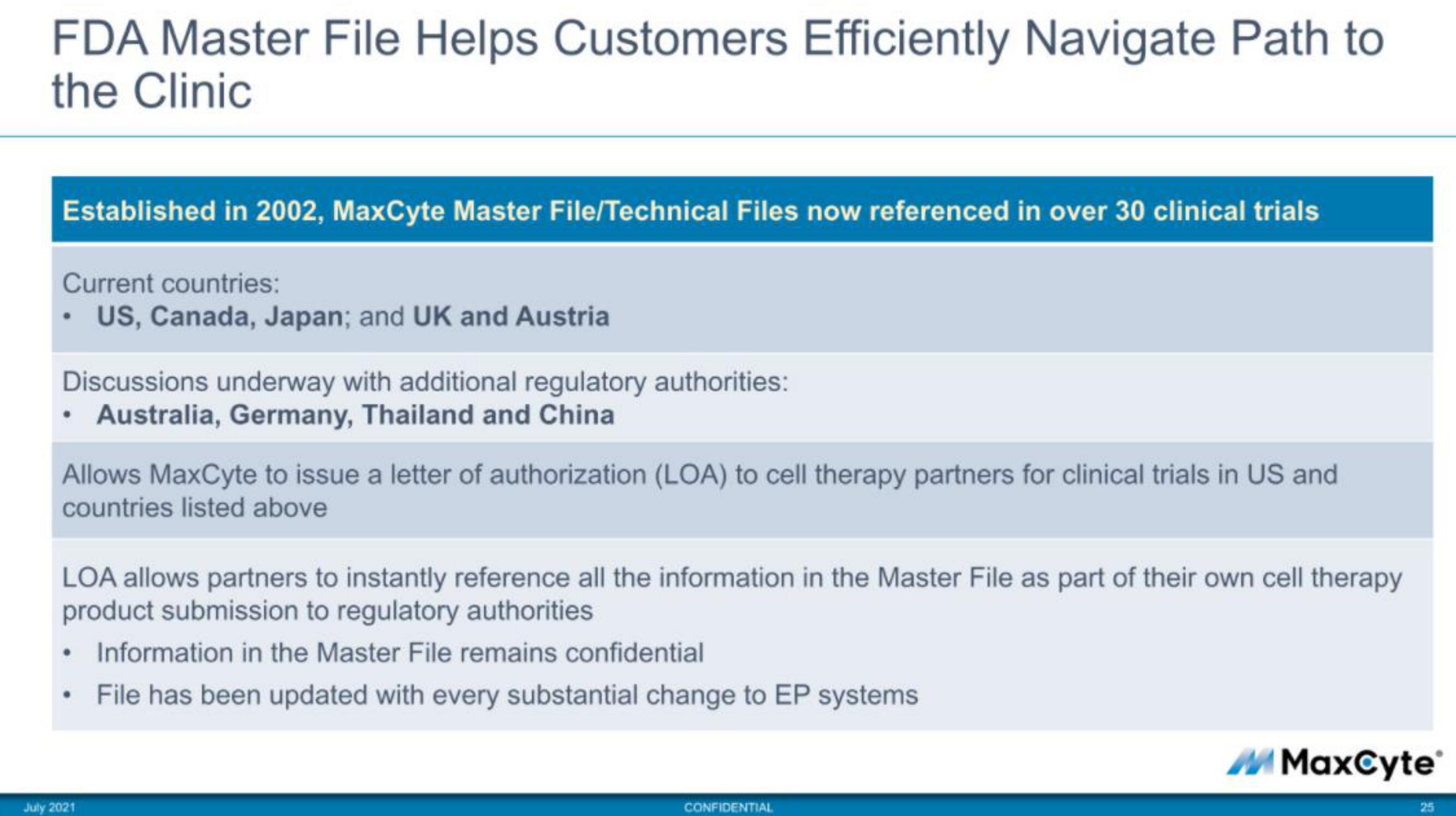
FDA Master File Helps Customers Efficiently Navigate Path to
the Clinic
Established in 2002, MaxCyte Master File/Technical Files now referenced in over 30 clinical trials
Current countries:
• US, Canada, Japan; and UK and Austria
Discussions underway with additional regulatory authorities:
Australia, Germany, Thailand and China
Allows MaxCyte to issue a letter of authorization (LOA) to cell therapy partners for clinical trials in US and
countries listed above
LOA allows partners to instantly reference all the information in the Master File as part of their own cell therapy
product submission to regulatory authorities
July 2021
Information in the Master File remains confidential
File has been updated with every substantial change to EP systems
CONFIDENTIAL
M MaxCyteView entire presentation