23andMe Investor Presentation Deck
1
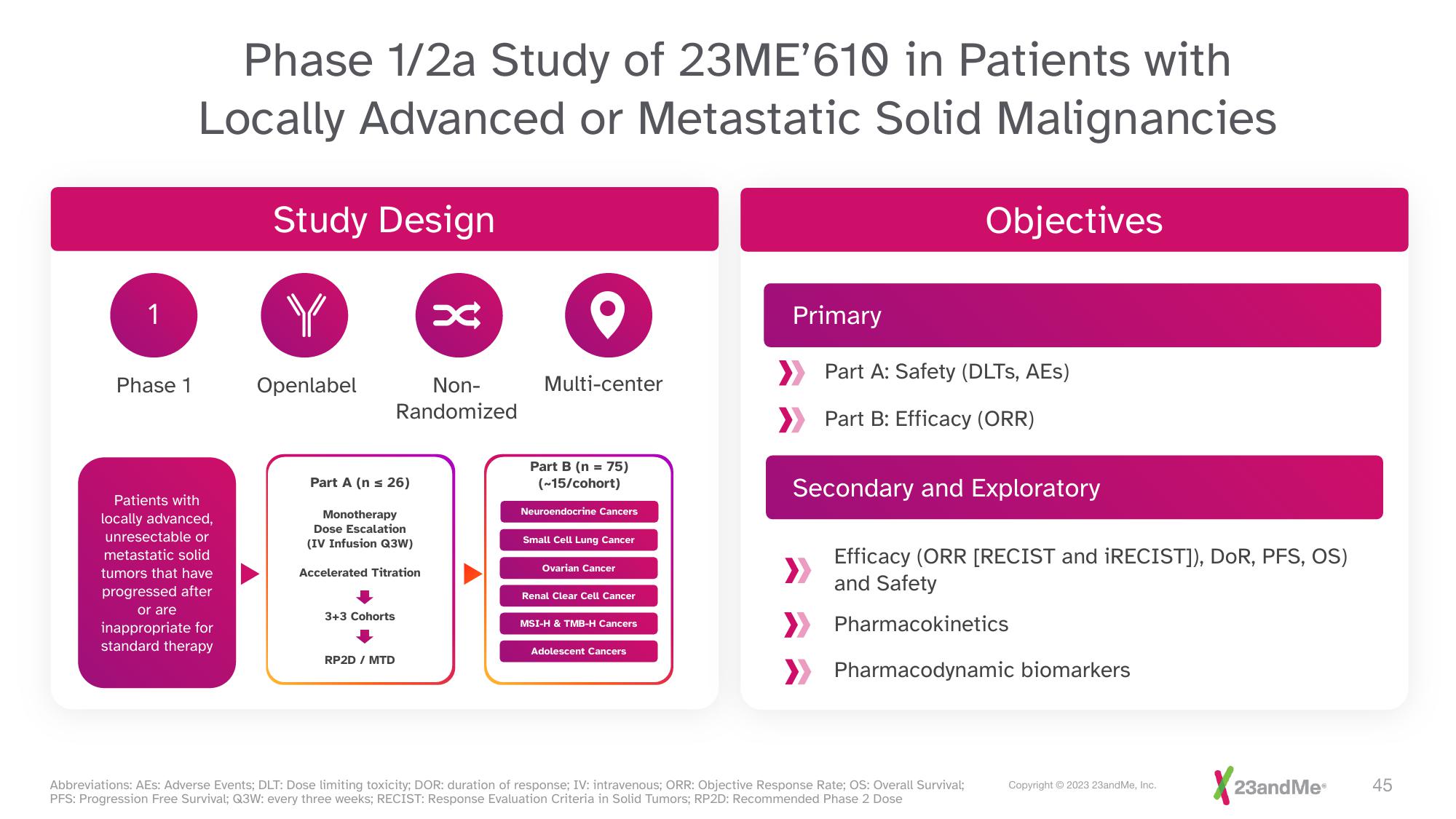
Phase 1
Phase 1/2a Study of 23ME'610 in Patients with
Locally Advanced or Metastatic Solid Malignancies
Patients with
locally advanced,
unresectable or
metastatic solid
tumors that have
progressed after
or are
inappropriate for
standard therapy
Study Design
Y
Openlabel
Part A (n = 26)
Monotherapy
Dose Escalation
(IV Infusion Q3W)
Accelerated Titration
3+3 Cohorts
RP2D / MTD
x
Non-
Randomized
Multi-center
Part B (n = 75)
(~15/cohort)
Neuroendocrine Cancers
Small Cell Lung Cancer
Ovarian Cancer
Renal Clear Cell Cancer
MSI-H & TMB-H Cancers
Adolescent Cancers
Primary
Objectives
Part A: Safety (DLTS, AES)
Part B: Efficacy (ORR)
Secondary and Exploratory
Efficacy (ORR [RECIST and iRECIST]), DOR, PFS, OS)
and Safety
> Pharmacokinetics
Pharmacodynamic biomarkers
Abbreviations: AEs: Adverse Events; DLT: Dose limiting toxicity; DOR: duration of response; IV: intravenous; ORR: Objective Response Rate; OS: Overall Survival;
PFS: Progression Free Survival; Q3W: every three weeks; RECIST: Response Evaluation Criteria in Solid Tumors; RP2D: Recommended Phase 2 Dose
Copyright © 2023 23and Me, Inc.
23andMe 45View entire presentation