AstraZeneca Results Presentation Deck
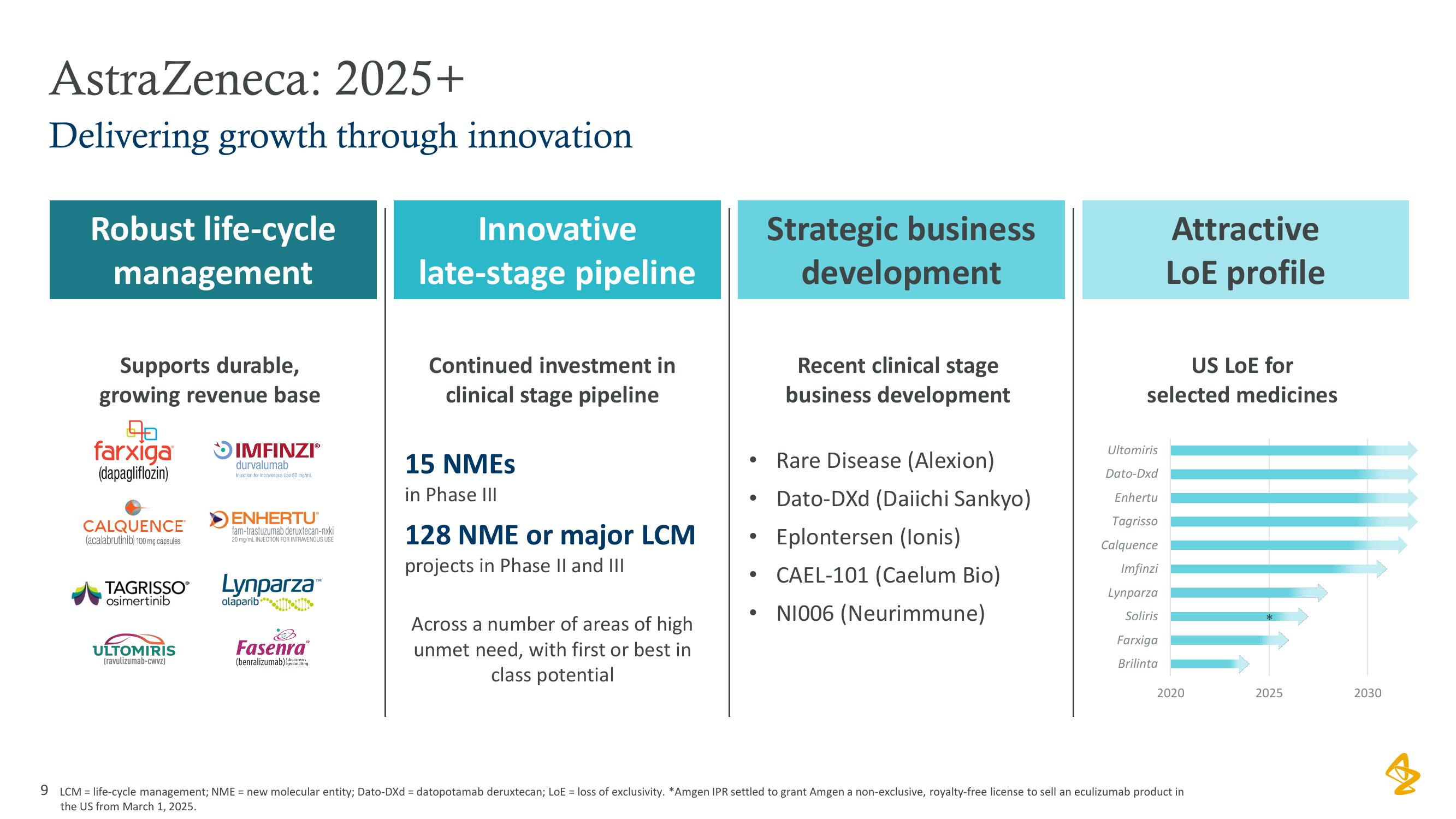
AstraZeneca: 2025+
Delivering growth through innovation
Robust life-cycle
management
Supports durable,
growing revenue base
90
farxiga
(dapagliflozin)
CALQUENCE®
(acalabrutinib) 100 mg capsules
TAGRISSO
osimertinib
ULTOMIRIS
(ravulizumab-cwvZ]
SIMFINZIⓇ
durvalumab
Injection for Intravenous Use 50 mg/ml
DENHERTUⓇ
fam-trastuzumab deruxtecan-nxki
20 mg/mL INJECTION FOR INTRAVENOUS USE
Lynparza
olaparib
Fasenra
(benralizumab) on 30 mg
Innovative
late-stage pipeline
Continued investment in
clinical stage pipeline
15 NMES
in Phase III
128 NME or major LCM
projects in Phase II and III
Across a number of areas of high
unmet need, with first or best in
class potential
Strategic business
development
●
Recent clinical stage
business development
• Rare Disease (Alexion)
Dato-DXd (Daiichi Sankyo)
Eplontersen (lonis)
• CAEL-101 (Caelum Bio)
●
NI006 (Neurimmune)
Attractive
LoE profile
US LOE for
selected medicines
Ultomiris
Dato-Dxd
Enhertu
Tagrisso
Calquence
Imfinzi
Lynparza
Soliris
Farxiga
Brilinta
2020
9 LCM = life-cycle management; NME = new molecular entity; Dato-DXd = datopotamab deruxtecan; LoE = loss of exclusivity. *Amgen IPR settled to grant Amgen a non-exclusive, royalty-free license to sell an eculizumab product in
the US from March 1, 2025.
2025
2030
BView entire presentation