Bionomics Results Presentation Deck
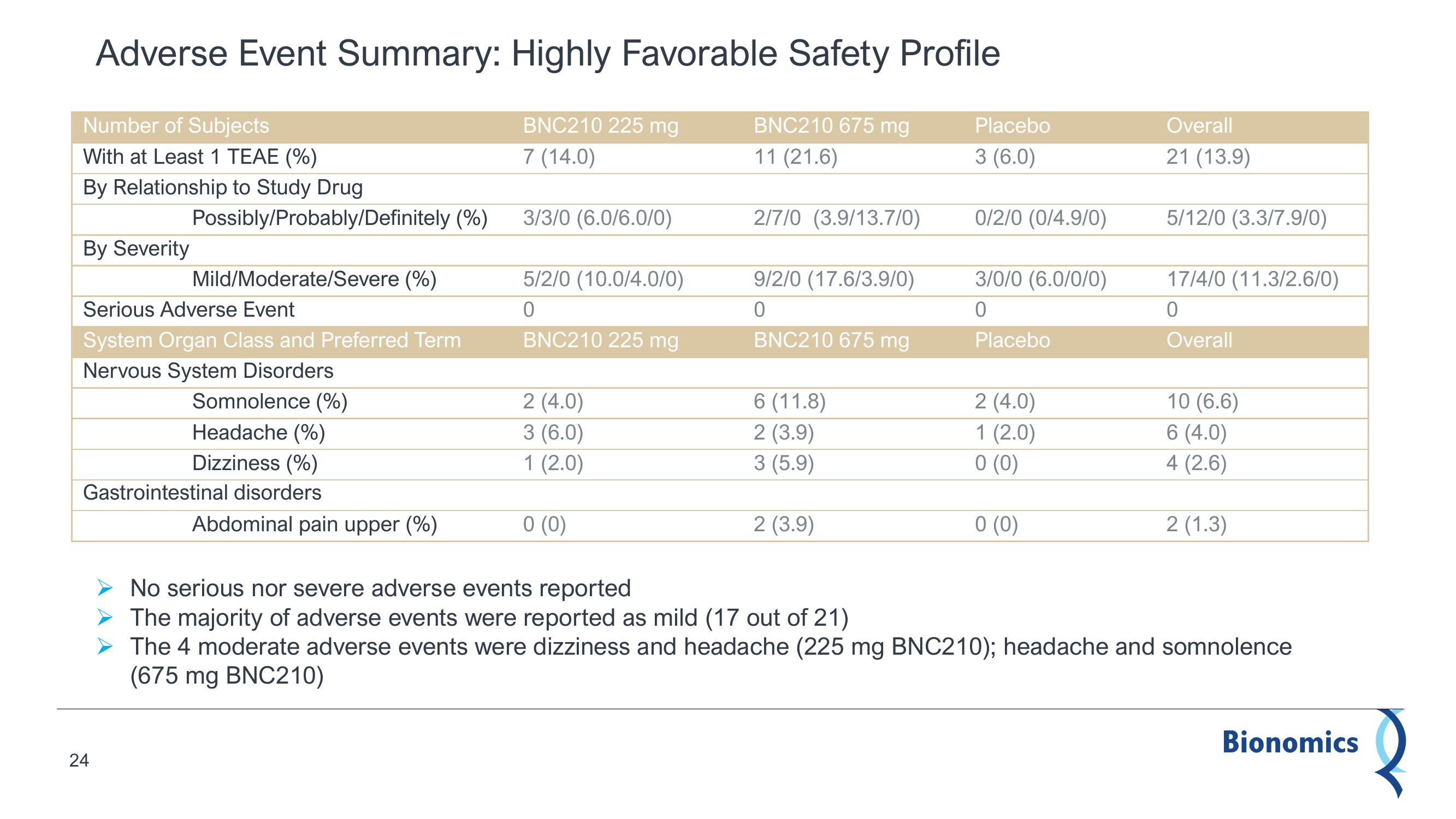
Adverse Event Summary: Highly Favorable Safety Profile
BNC210 225 mg
7 (14.0)
BNC210 675 mg
11 (21.6)
Number of Subjects
With at Least 1 TEAE (%)
By Relationship to Study Drug
By Severity
Possibly/Probably/Definitely (%)
24
Mild/Moderate/Severe (%)
Serious Adverse Event
System Organ Class and Preferred Term
Nervous System Disorders
Somnolence (%)
Headache (%)
Dizziness (%)
Gastrointestinal disorders
Abdominal pain upper (%)
3/3/0 (6.0/6.0/0)
5/2/0 (10.0/4.0/0)
0
BNC210 225 mg
2 (4.0)
3 (6.0)
1 (2.0)
0 (0)
2/7/0 (3.9/13.7/0)
9/2/0 (17.6/3.9/0)
0
BNC210 675 mg
6 (11.8)
2 (3.9)
3 (5.9)
2 (3.9)
Placebo
3 (6.0)
0/2/0 (0/4.9/0)
3/0/0 (6.0/0/0)
0
Placebo
2 (4.0)
1 (2.0)
0 (0)
0 (0)
Overall
21 (13.9)
5/12/0 (3.3/7.9/0)
17/4/0 (11.3/2.6/0)
0
Overall
10 (6.6)
6 (4.0)
4 (2.6)
2 (1.3)
No serious nor severe adverse events reported
The majority of adverse events were reported as mild (17 out of 21)
➤ The 4 moderate adverse events were dizziness and headache (225 mg BNC210); headache and somnolence
(675 mg BNC210)
BionomicsView entire presentation