Bionomics Results Presentation Deck
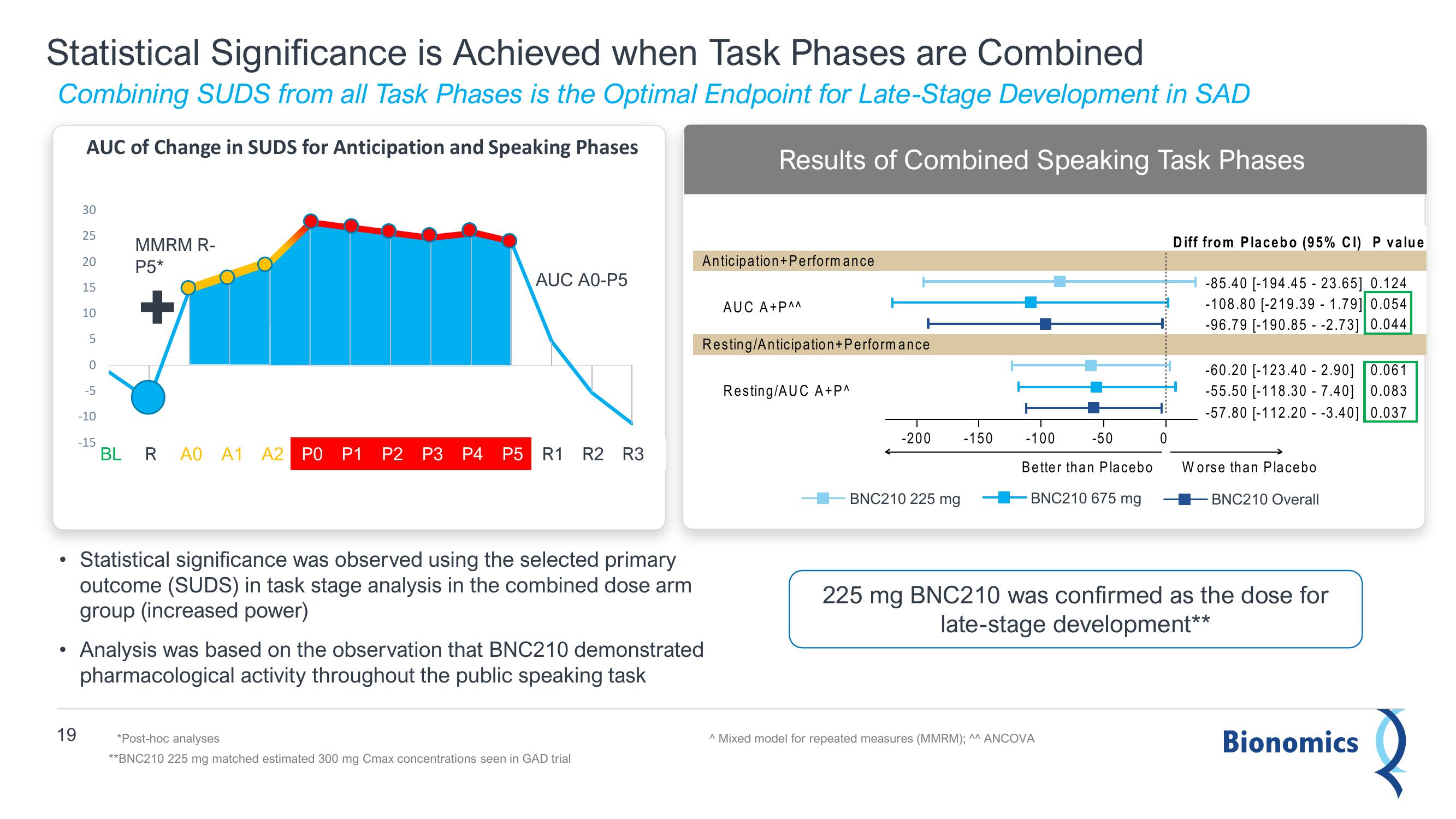
Statistical Significance is Achieved when Task Phases are Combined
Combining SUDS from all Task Phases is the Optimal Endpoint for Late-Stage Development in SAD
AUC of Change in SUDS for Anticipation and Speaking Phases
Results of Combined Speaking Task Phases
●
30
25
19
20
15
10
5
-5
-10
MMRM R-
P5*
AUC A0-P5
• Statistical significance was observed using the selected primary
outcome (SUDS) in task stage analysis in the combined dose arm
group (increased power)
-15
BL R A0 A1 A2 PO P1 P2 P3 P4 P5 R1 R2 R3
Anticipation+Performance
*Post-hoc analyses
**BNC210 225 mg matched estimated 300 mg Cmax concentrations seen in GAD trial
Analysis was based on the observation that BNC210 demonstrated
pharmacological activity throughout the public speaking task
AUC A+P^^
Resting/Anticipation+Performance
H
Resting/AUC A+P^
-200
BNC210 225 mg
-150
-100
Better than Placebo
BNC210 675 mg
-50
^ Mixed model for repeated measures (MMRM); ^^ ANCOVA
0
Diff from Placebo (95% Cl) P value
-85.40 [-194.45 - 23.65] 0.124
-108.80 [-219.39 - 1.79] 0.054
-96.79 [-190.85 -2.73] 0.044
-60.20 [-123.40 - 2.90] 0.061
-55.50 [-118.30 - 7.40] 0.083
-57.80 [-112.20 - -3.40] 0.037
Worse than Placebo
225 mg BNC210 was confirmed as the dose for
late-stage development*
BNC210 Overall
.**
BionomicsView entire presentation