Bionomics Results Presentation Deck
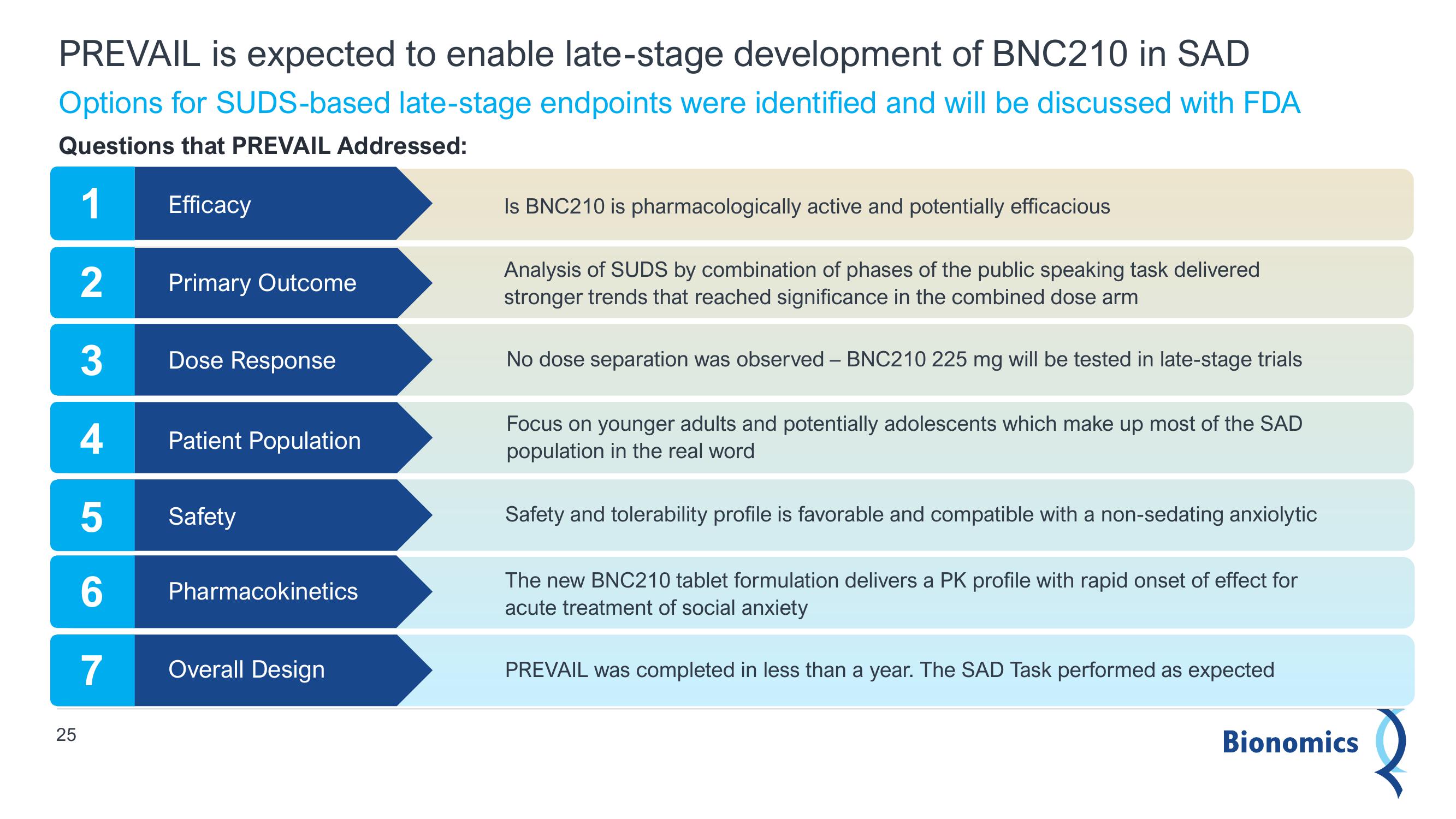
PREVAIL is expected to enable late-stage development of BNC210 in SAD
Options for SUDS-based late-stage endpoints were identified and will be discussed with FDA
Questions that PREVAIL Addressed:
1
2
3
4
5
6
7
25
Efficacy
Primary Outcome
Dose Response
Patient Population
Safety
Pharmacokinetics
Overall Design
Is BNC210 is pharmacologically active and potentially efficacious
Analysis of SUDS by combination of phases of the public speaking task delivered
stronger trends that reached significance in the combined dose arm
No dose separation was observed - BNC210 225 mg will be tested in late-stage trials
Focus on younger adults and potentially adolescents which make up most of the SAD
population in the real word.
Safety and tolerability profile is favorable and compatible with a non-sedating anxiolytic
The new BNC210 tablet formulation delivers a PK profile with rapid onset of effect for
acute treatment of social anxiety
PREVAIL was completed in less than a year. The SAD Task performed as expected
BionomicsView entire presentation