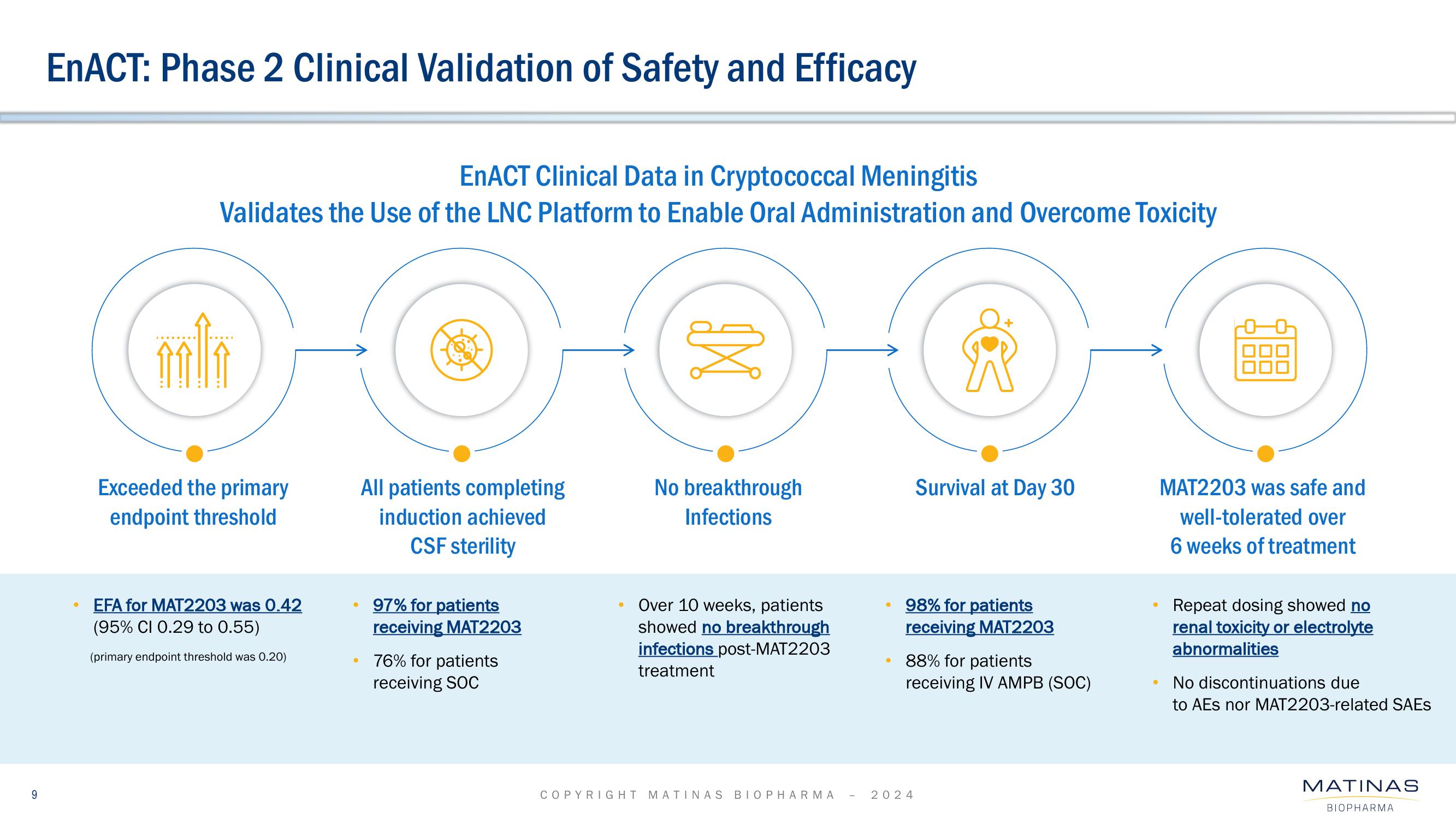
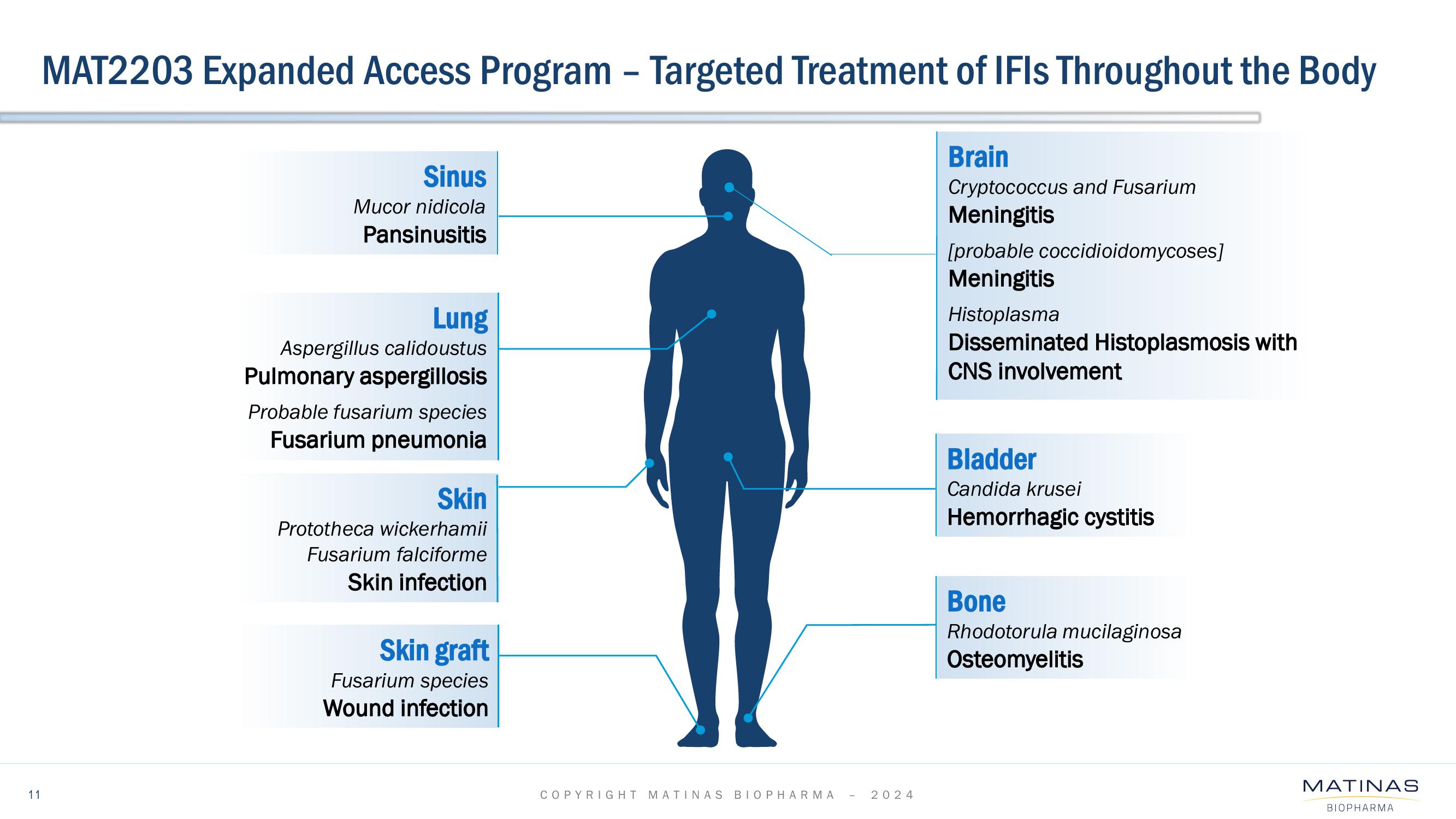
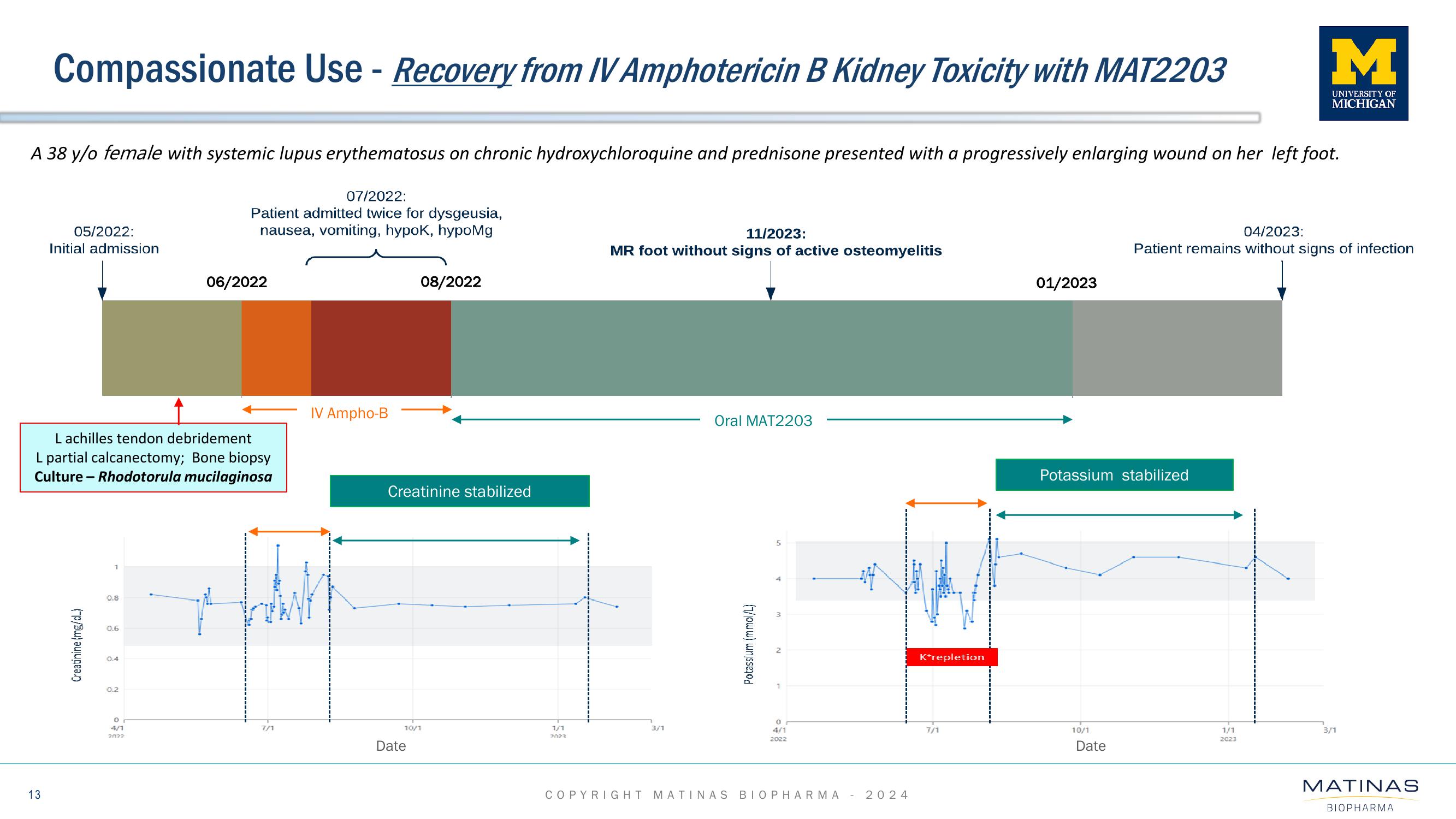
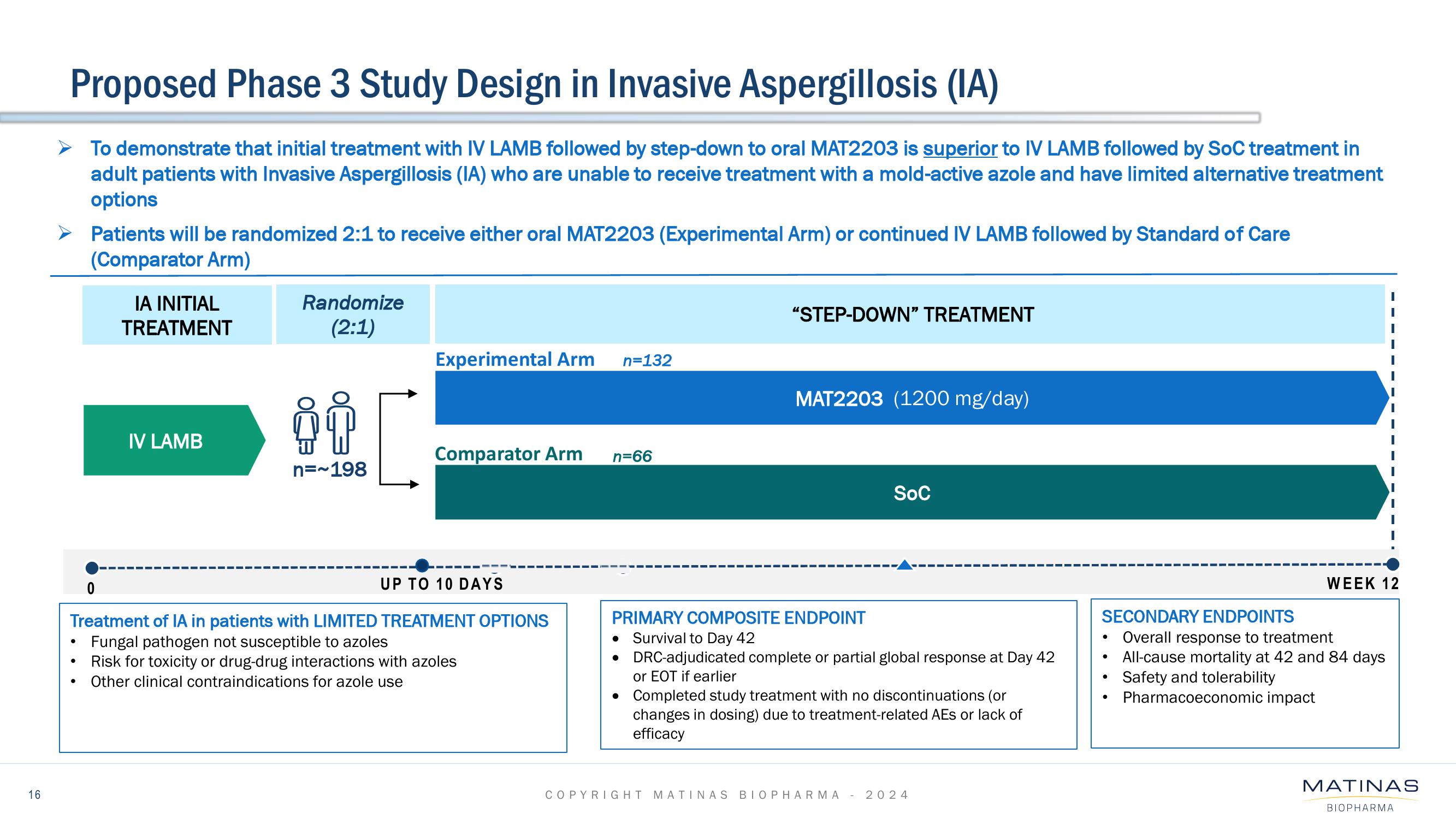
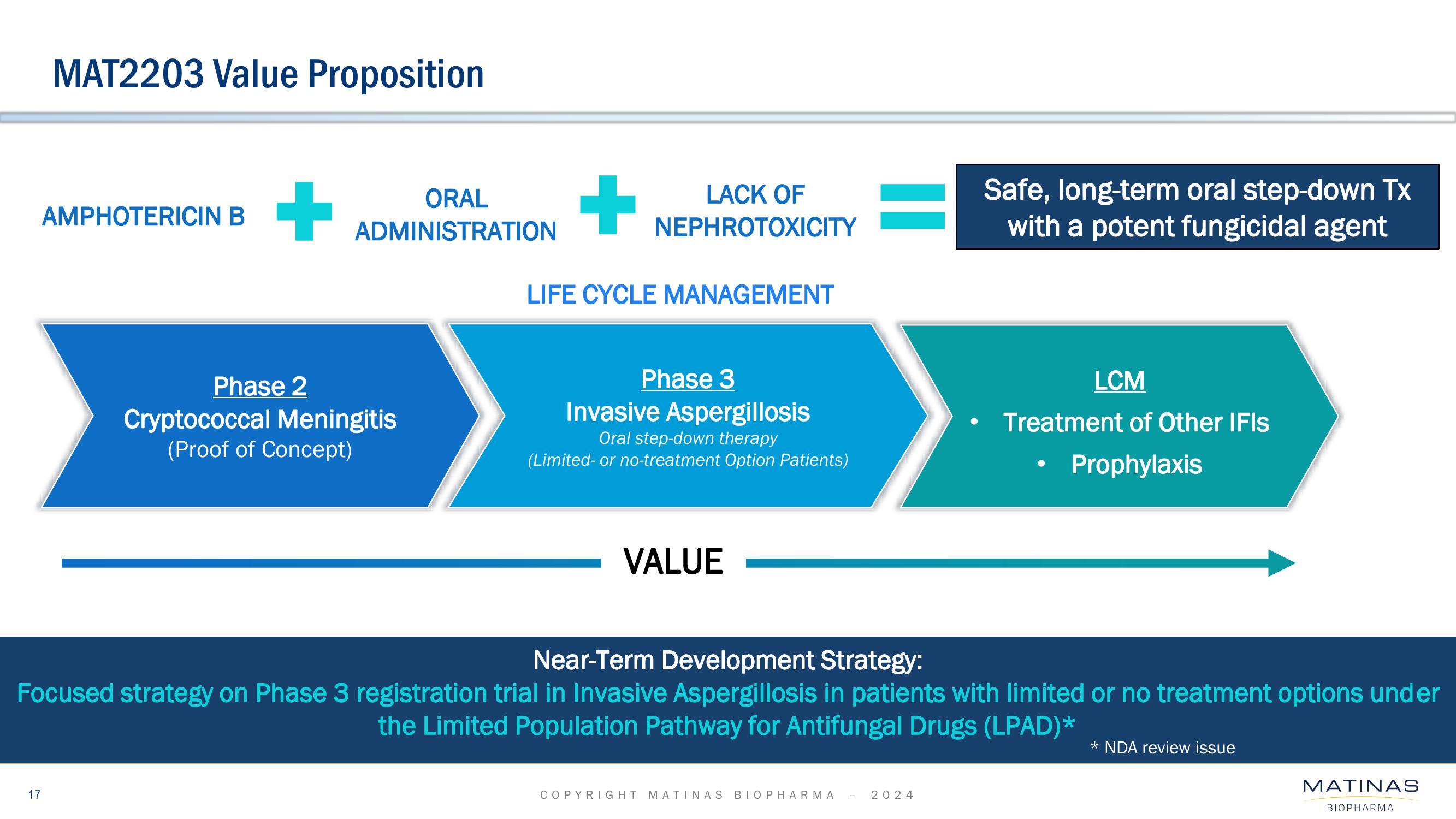
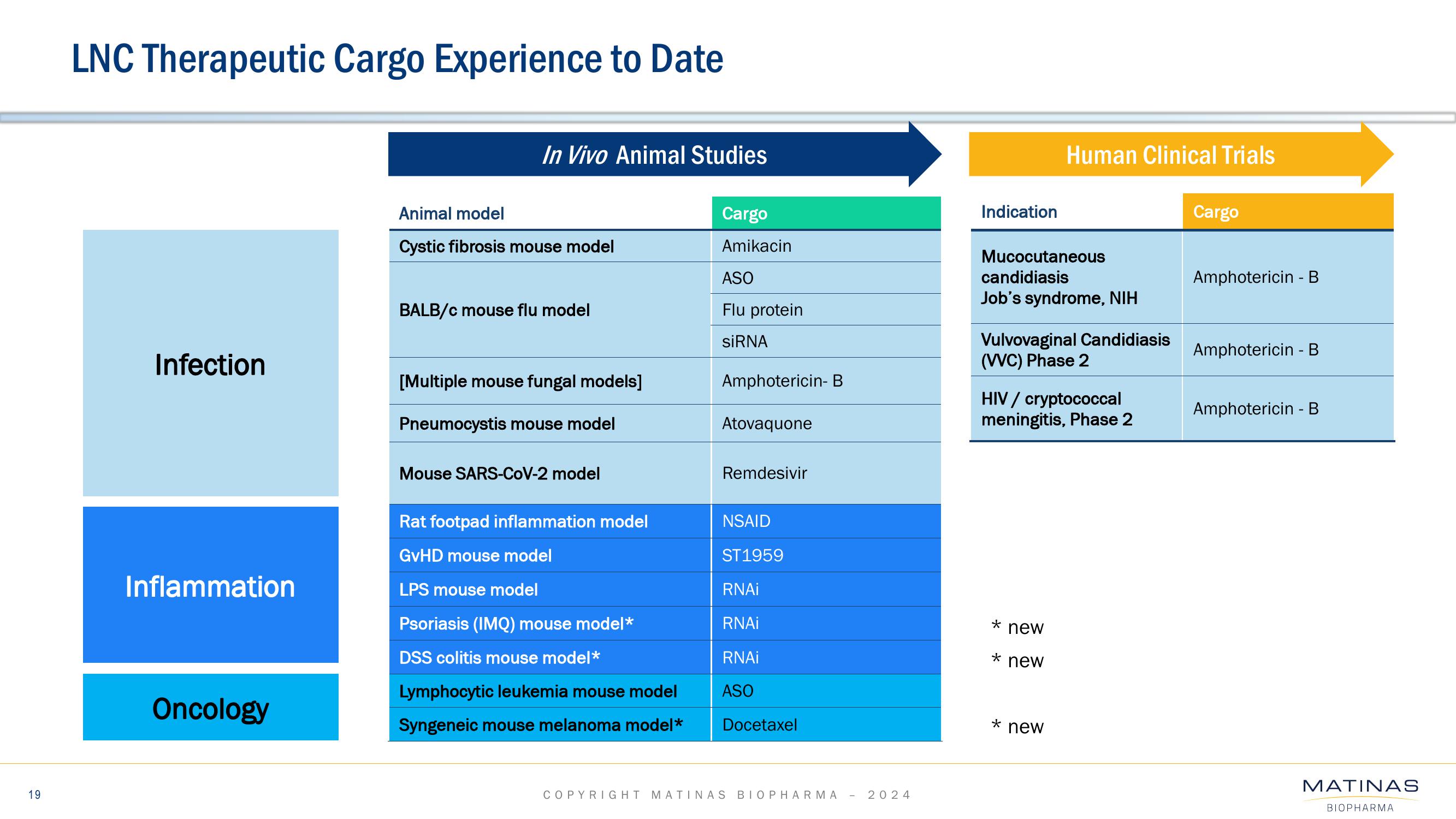
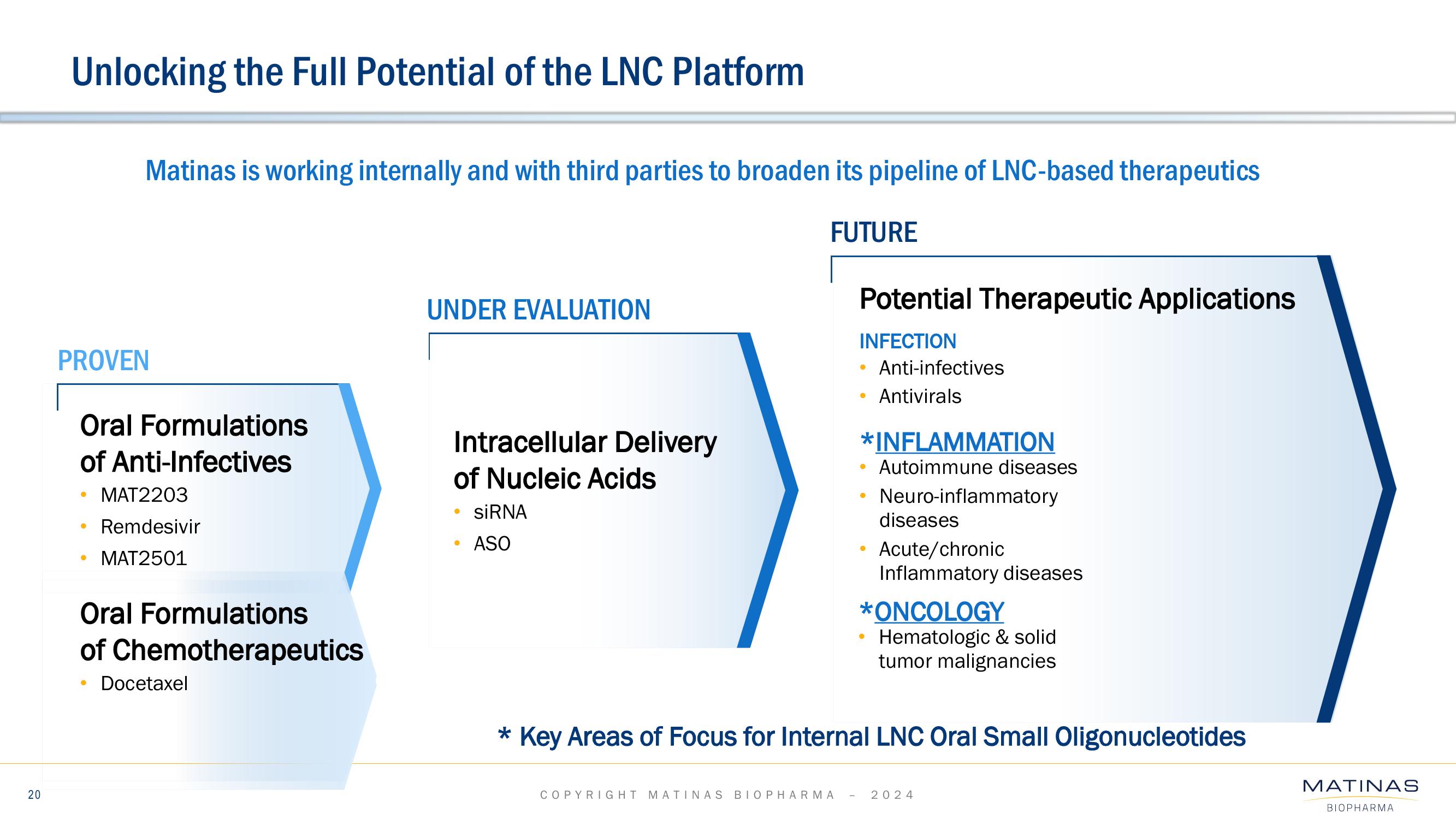
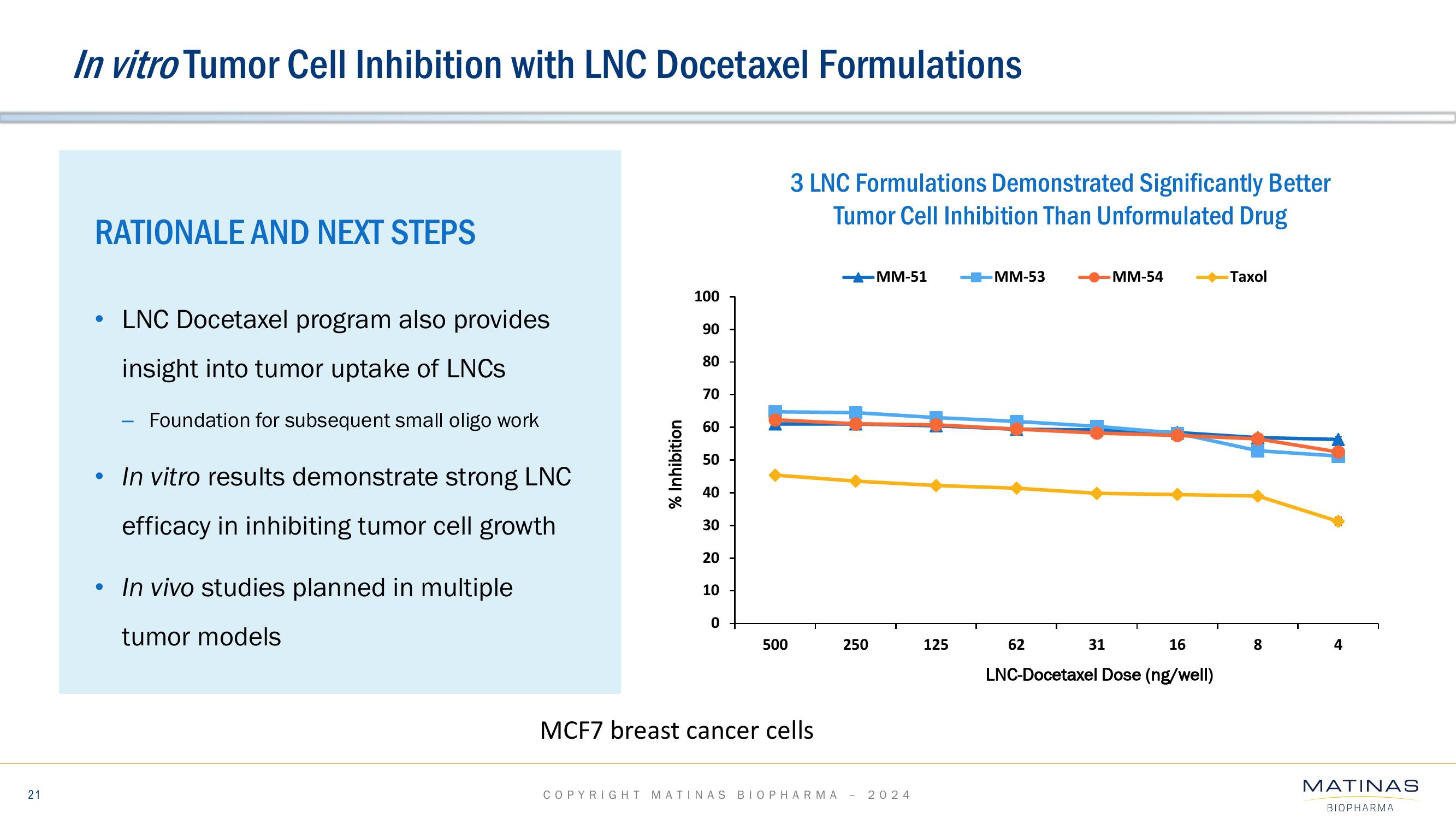
Matinas BioPharma Investor Presentation Deck
Made public by
Matinas Biopharma
sourced by PitchSend
Creator
matinas-biopharma
Category
Healthcare
Published
January 2024
Slides
Transcriptions
Download to PowerPoint
Download presentation as an editable powerpoint.
Related