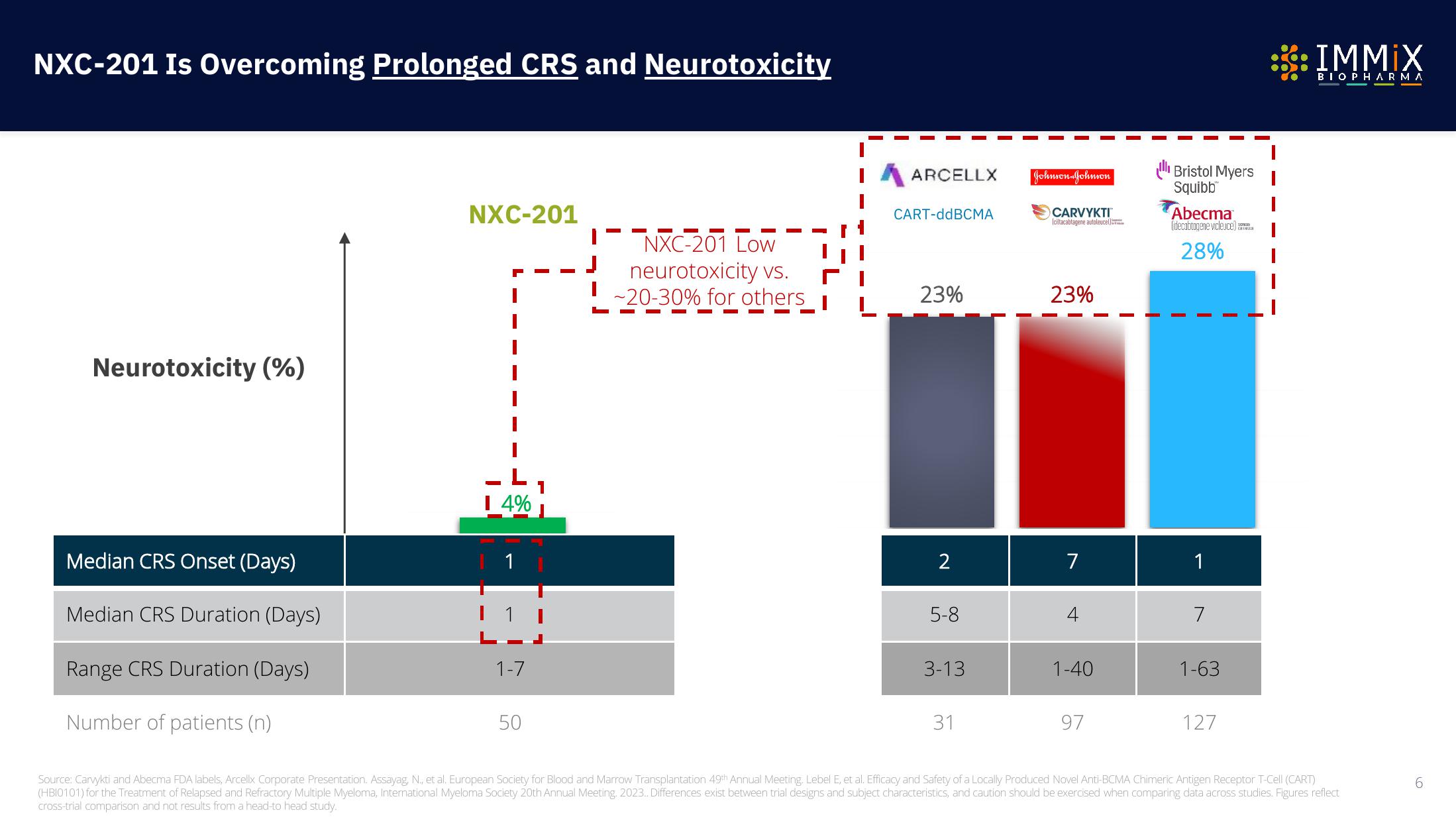
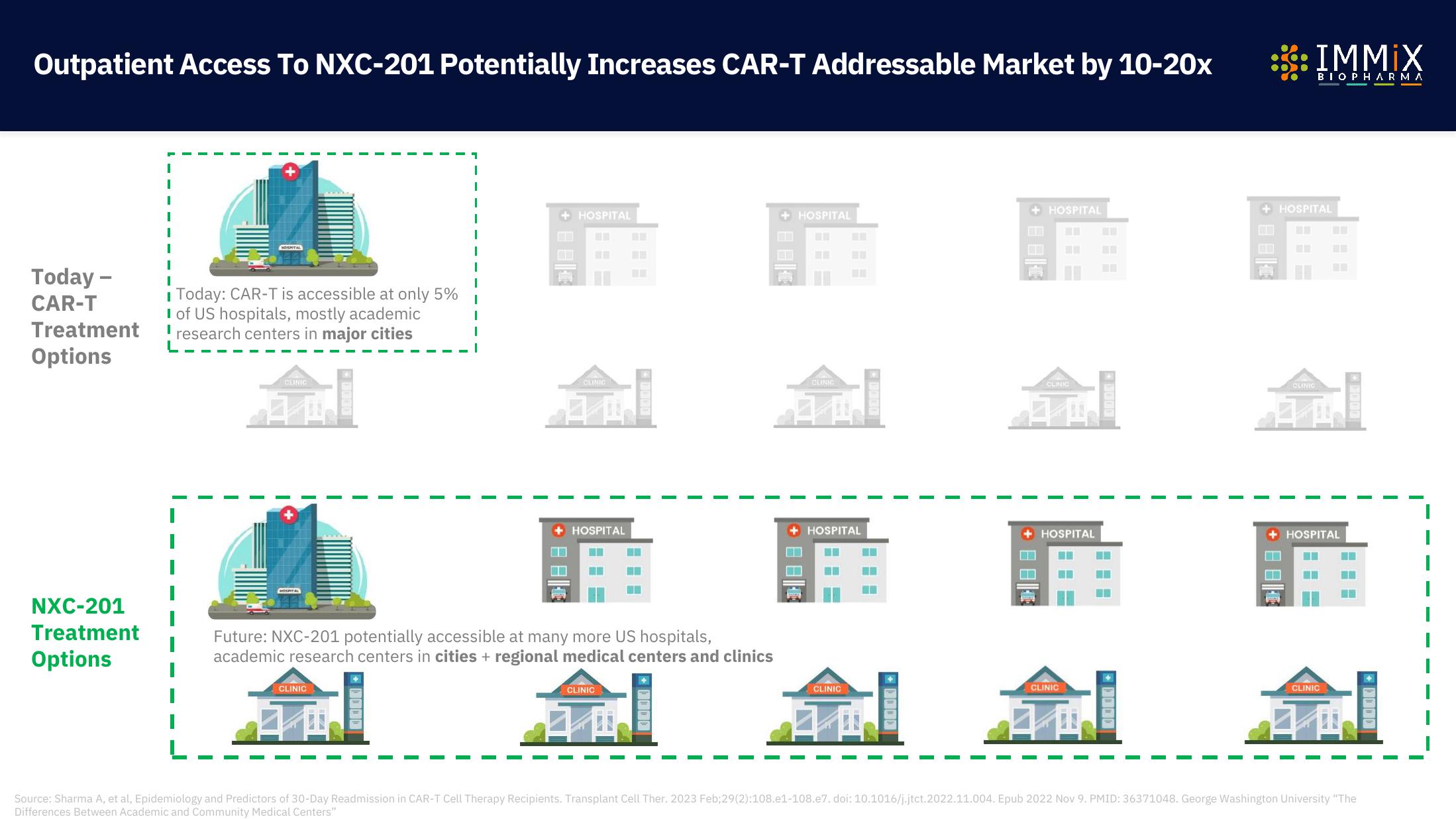
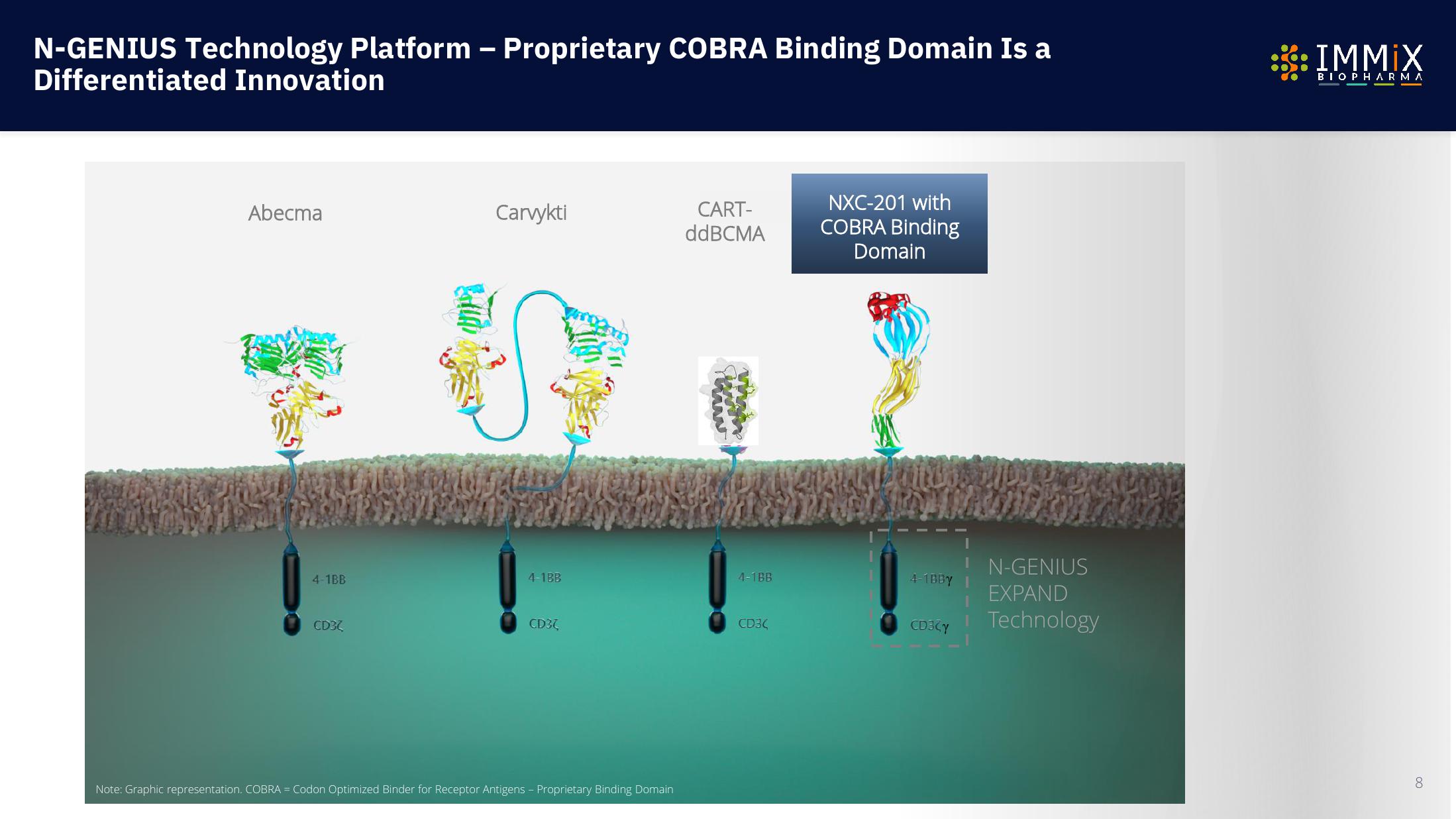
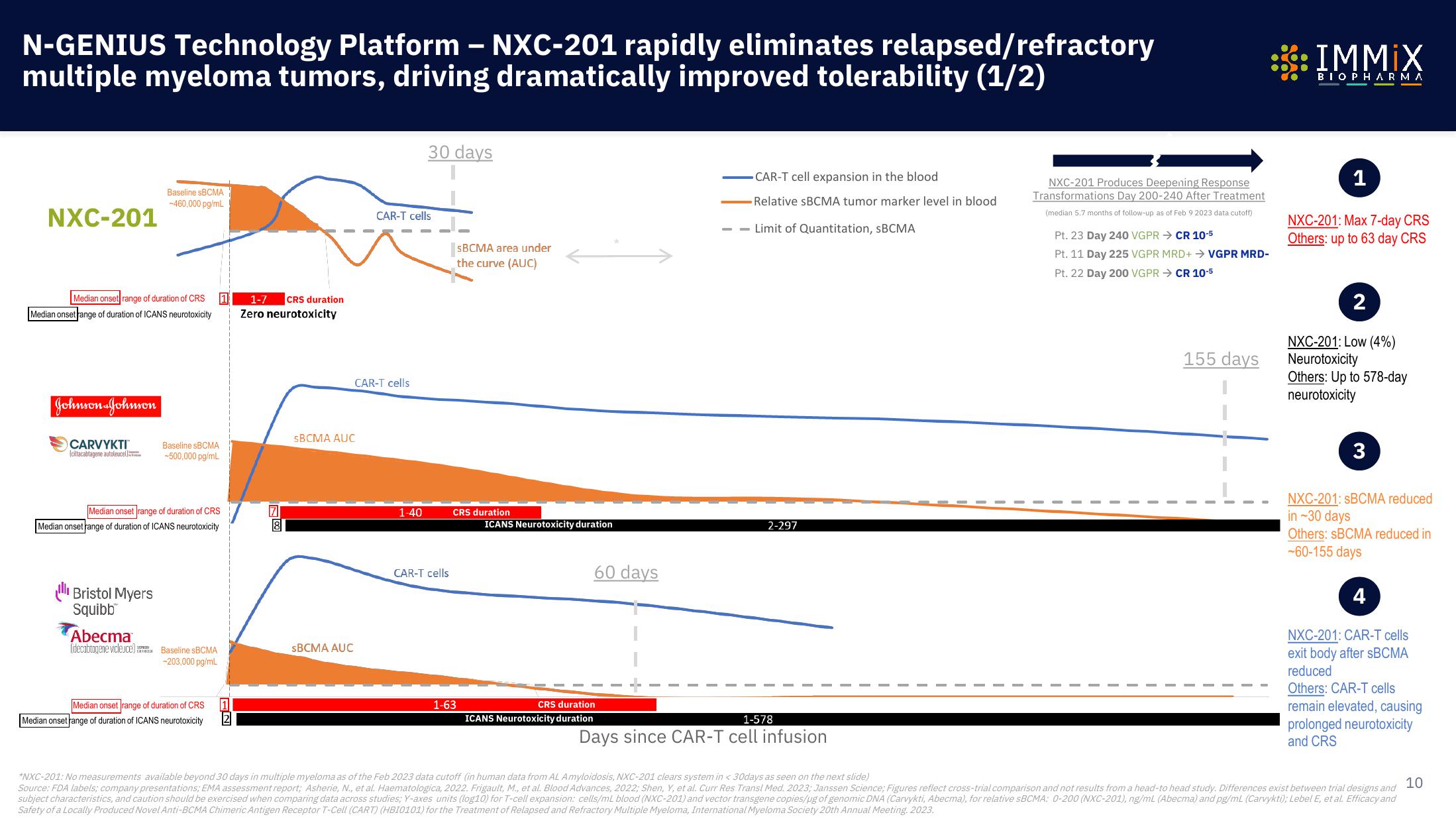
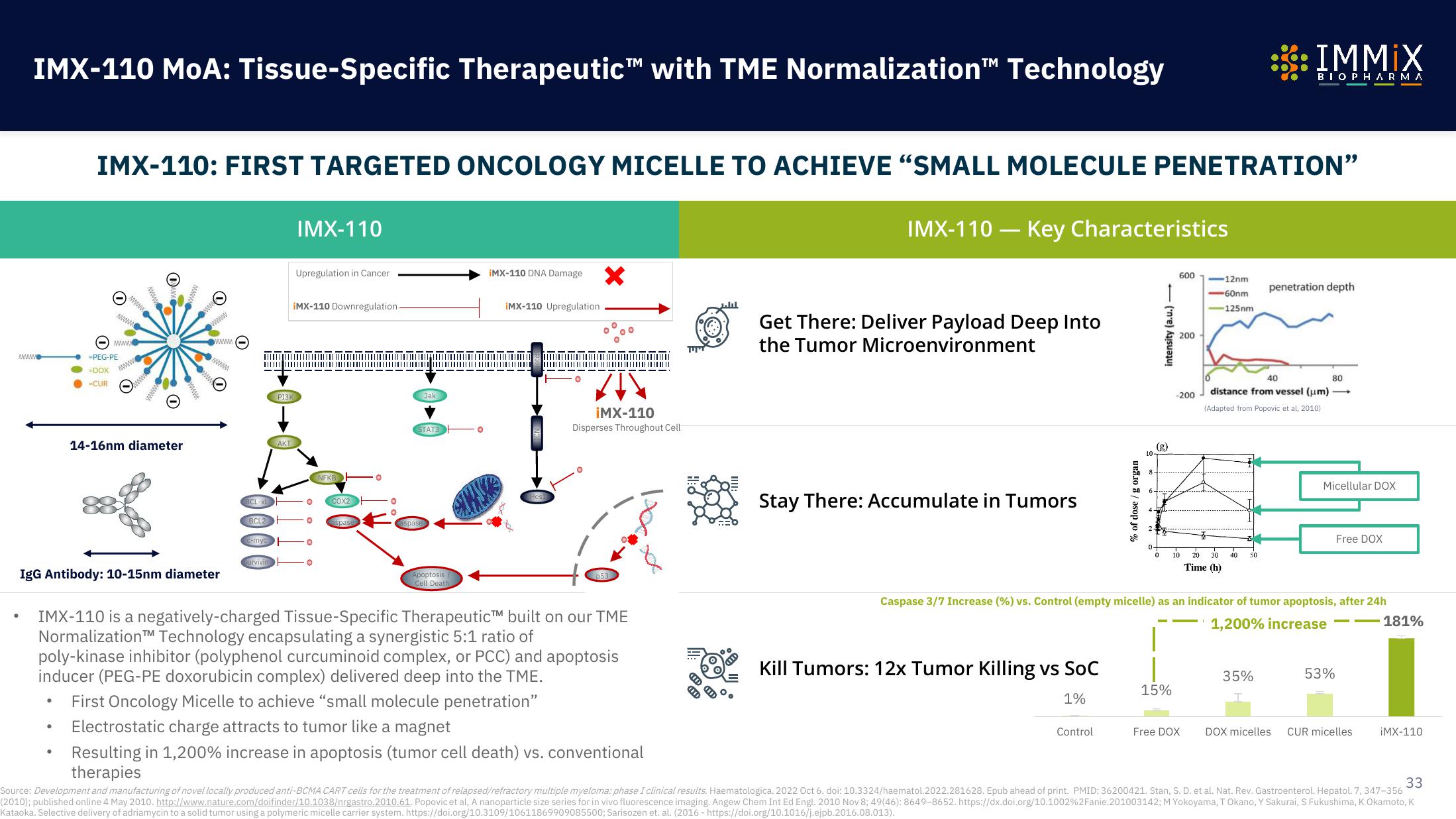
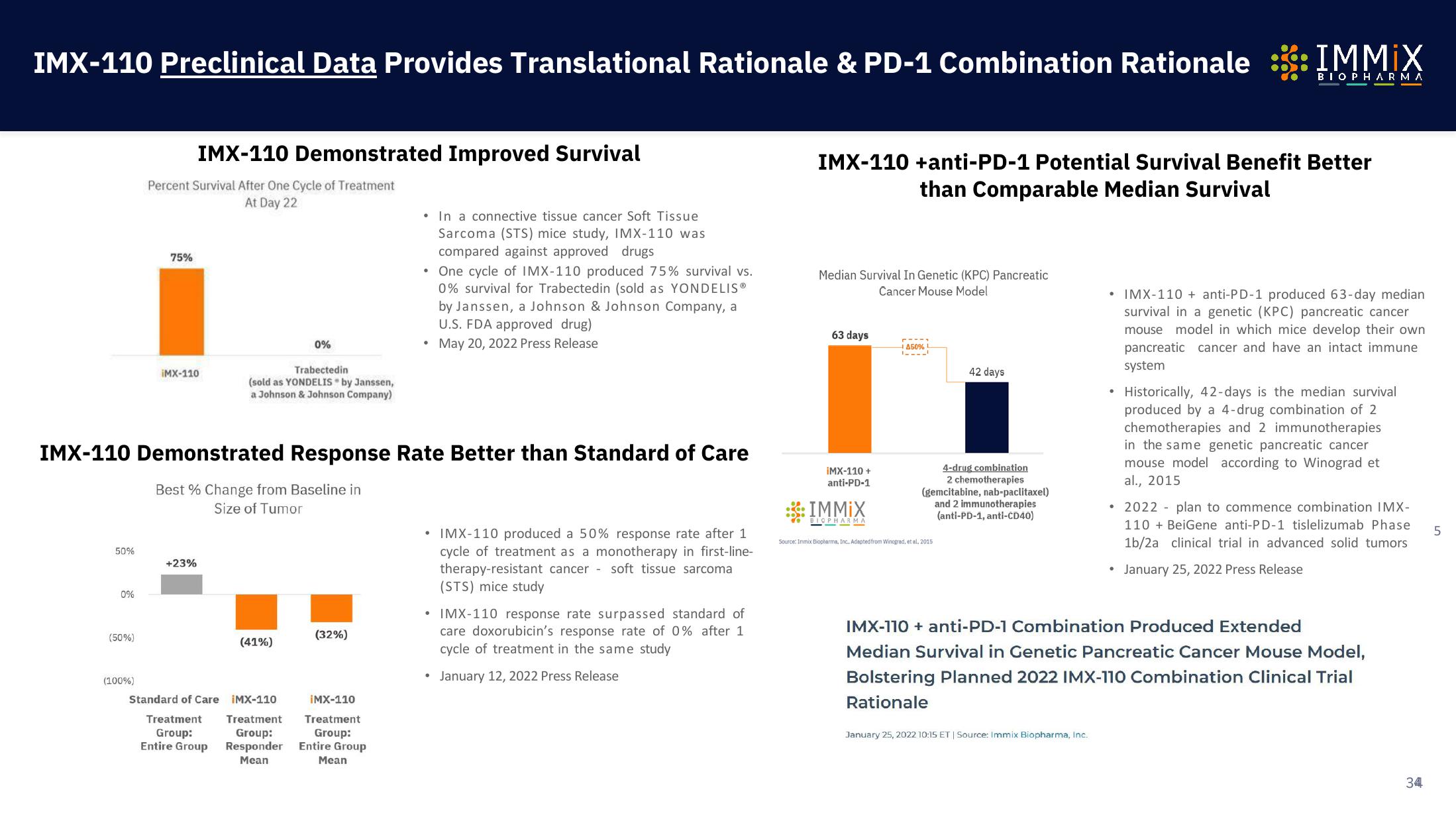
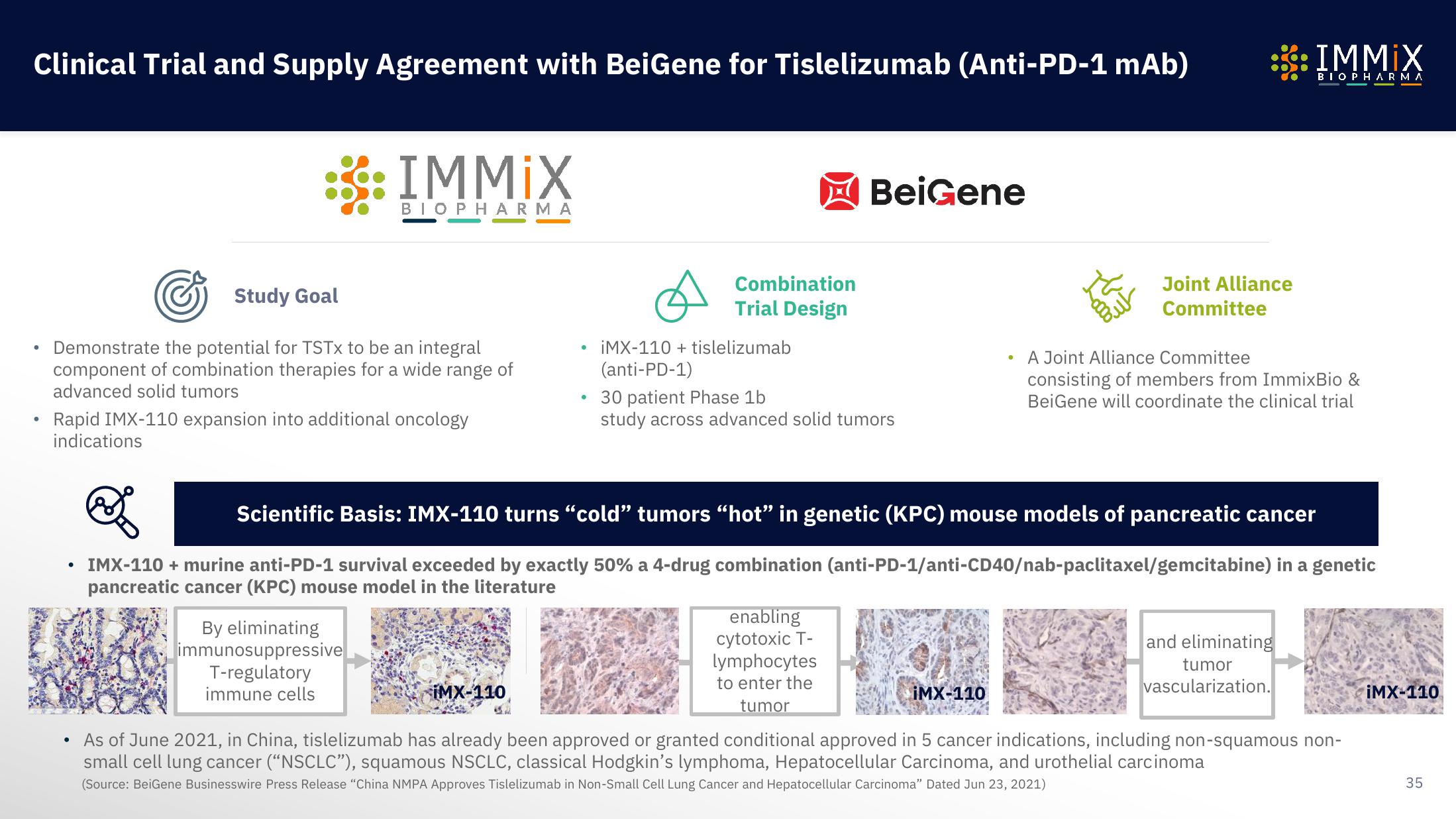
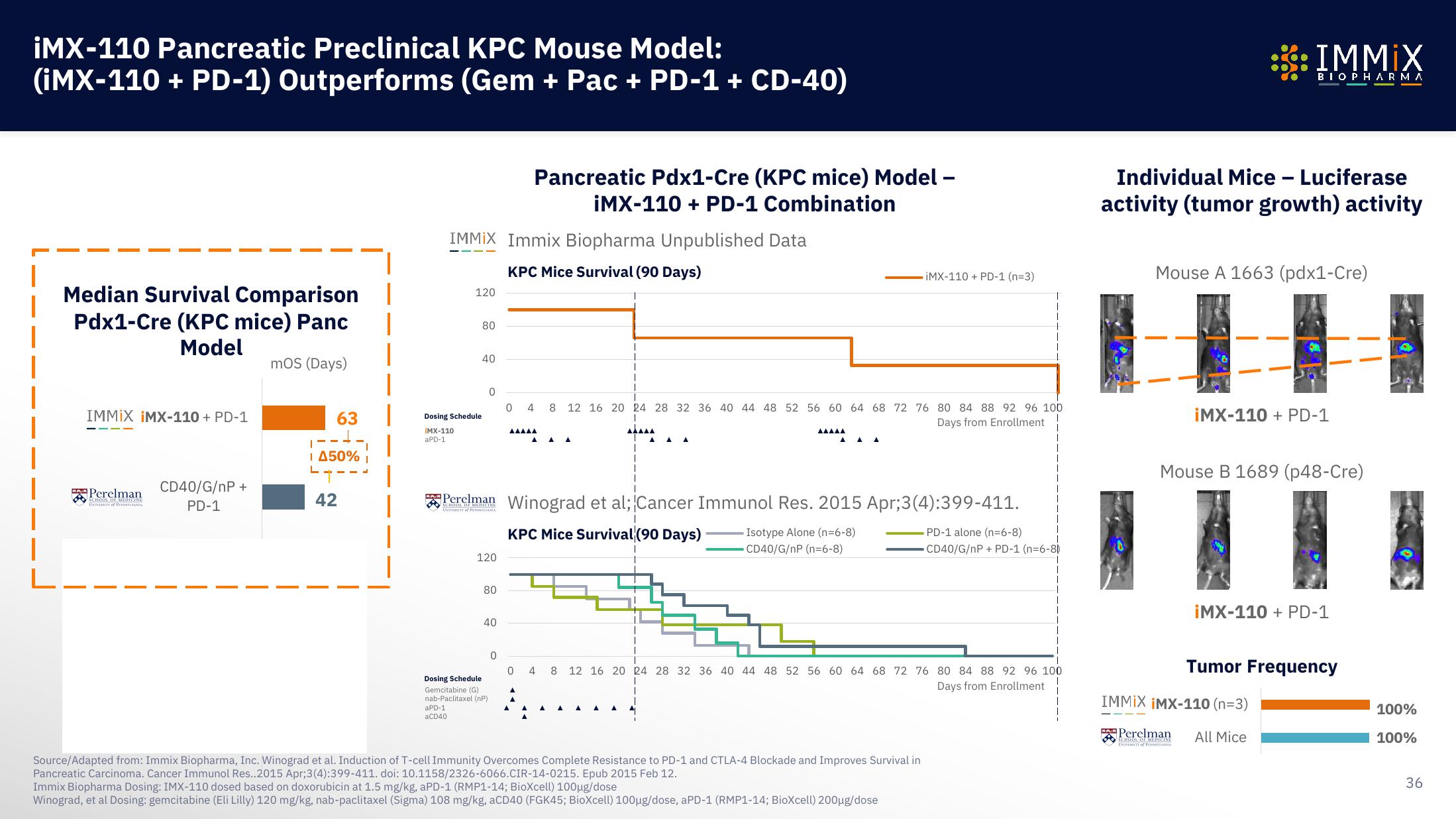
Immix Biopharma Investor Presentation Deck
Made public by
Immix Biopharma
sourced by PitchSend
Creator
immix-biopharma
Category
Healthcare
Published
October 2023
Slides
Transcriptions
Download to PowerPoint
Download presentation as an editable powerpoint.
Related